In a paper, to be featured in the April 2021 edition of Cell, a multinational research team led by the Prosser lab at the University of Toronto and colleagues at RIKEN in Japan, reveal key steps associated with the activation of the human adenosine A2A receptor (A2AR). A2AR belongs to a superfamily of 7-transmembrane receptors called G protein-coupled receptors (GPCRs), which engage G proteins and regulate cell signaling. One-third of all FDA-approved drugs target GPCRs and the mechanistic principles governing this pharmacology is of great interest to the scientific community.
The sole first author on this paper, Kate Huang, expressed and reconstituted this human receptor in synthetic lipid bilayers. Complexation with the heterotrimeric G protein was an astronomical feat considering the amount of materials needed for NMR experiments and the fact that yeast, E. coli, and insect cells were all utilized to produce the proteins needed to assemble the complex.
The research identifies key receptor states associated with the signalling pathway, including a precoupled state which enables G protein binding, and two active states which are stabilized by partial or full-agonist drugs. Work by the RIKEN and Tokyo Institute of Technology team builds upon the biophysical studies and observation of these states by predicting an allosteric network extending across the receptor-G protein signaling complex. Much of this conformational detail is currently lacking from more traditional X-ray crystallography or cryo-electron microscopy methods and is critical to understanding pharmacology.
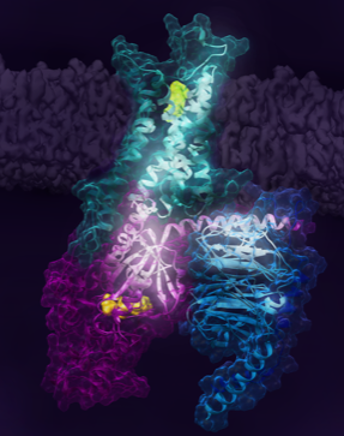
There are over 800 GPCRs in the human genome and many share a similar topology and mechanism of action. In most cases, GPCRs are situated in the plasma membrane surrounding the cell, while the drug or ligand that acts on the receptor binds to an extracellular pocket. Signal is then transduced across the protein, resulting in complexation with binding partners on the cell interior and the initiation of downstream pathways. Sensory signalling (sight, smell, taste, pain) is mediated by GPCRs. This also makes GPCRs useful in pharmacology since the drug need not enter the cell, in many cases. While recent advances in X-ray crystallography and Cryo-electron microscopy have generated high-resolution atomistic 3D structures of many GPCRs, these techniques could not capture the dynamical nature of GPCRs which is central to their function and the associated pharmacology.
In this study, researchers used Nuclear Magnetic Resonance (NMR) and computational methods to decipher changes in the conformational dynamics of the human adenosine A2A receptor as it navigates the activation pathway. GPCR signalling is known to involve a sequence of events where the receptor dynamically engages the G protein resulting in the egress of a nucleotide (GDP) from the G protein. The G protein is then activated after picking up GTP from the cell interior. The activated alpha subunit of the G protein is then free to dissociate and initiate downstream signalling. Here the researchers focused on biasing key states of the receptor by complexing it with G protein in the presence or the absence of nucleotide, as a function of drug. The resulting study provided a new way to view pharmacology and addressed longstanding questions regarding basal signalling, partial agonism, and pre-coupling in GPCRs.