A semester-long group project in CHM310 - The Fate and Toxicology of Organic Contaminants is the subject of a new article in the Journal of Chemical Education whose authors include the Department of Chemistry’s Jessica D’eon and Mima Staikova.
“Welcome to 310 Environmental Working Group! A Group Project That Places Students in the Role of Consultants Helping Businesses Choose the Most Climate Friendly Fluorinated Gas,” describes a group project in which teams of students assess the environmental friendliness of two fluorinated gases.
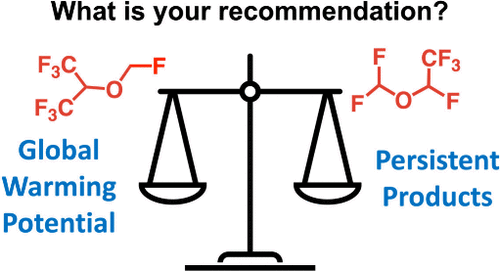
Assuming a consulting role for a hypothetical client, CHM310 students determined the global warming potential of the two chemicals together with their potential to produce persistent oxidation products. Students then sorted through sometimes-conflicting pieces of evidence, before making a final recommendation to their client as to which was the most environmentally friendly option, in a mock Board of Directors meeting.

The assignment was inspired some years ago, said Professor D'eon, by the work of Dr. Cora Young of York University, another of the paper’s co-authors. “I approached her with the idea of trying to get students to assess the potency of a greenhouse gas from first principles, which uses knowledge developed in our first and second year chemistry classes.”
D’eon was interested in guiding students through a process that brought them to answers that could be considered reasonable without being absolute, and then making recommendations to the assignment’s presumed client which balanced varying environmental considerations. The climate impacts of the Montreal Protocol, the 1987 global agreement to protect the stratospheric ozone layer by phasing out use of ozone-depleting substances, guided discussions in the class.
“The Montreal Protocol is a green chemistry success story that people don’t know enough about,” said D’eon. “We are using it to show students how the knowledge that they’re gaining has applications outside of the classroom."
D’eon explained that the Montreal Protocol controls chemicals in relation to their ability to disrupt the ozone layer and the climate system. This has resulted in significant improvements to these environmental metrics, but many of the newer generation materials degrade to produce trifluoroactic acid (TFA), an as-of-yet unregulated contaminant.
“TFA has no natural degradation routes,” said D’eon. “It’s incredibly persistent, it ends up in water and then just stays there. Because it is not acutely toxic, it is unregulated. The question is: Is that okay?”
In the CHM310 project, students use predictive tools to model the likely outcomes of a given chemical’s use. The result is not a definitive answer so much as a framework for thinking about the problem and making recommendations.
To characterize each chemical’s climate impact, students needed gas phase infrared (IR) spectra, which were predicted using quantum chemistry calculations.
“Using the knowledge that we generate in chemistry programs—in this case understanding organic reaction mechanisms and infrared spectroscopy--lets students imagine being a consultant and applying their breadth of knowledge. The project demonstrates how a student’s chemistry knowledge base can be used to develop a systems-wide perspective... visualizing how all the pieces work together.”
Mima Staikova, a Sessional Lecturer II at the Department of Chemistry, explained that the group project in CHM310 included a series of assignments addressing the different aspects of environmental consultant work.
“In assignment 2, students utilized quantum chemistry methods to calculate the IR spectra of a series of fluorocarbons, and then used them to calculate each chemical’s radiative efficiency, and from there their global warming potential.”
“Students in this class, and in chemistry courses in general, have experience recording and interpreting IR spectra using experimental methods,” Staikova explained. “As added learning experience this part of the project--calculating the IR spectra--showcases a quantum mechanical approach for predicting experimental properties. This also allows students to visualize the vibrational modes of each IR band, deepening their appreciation of what is physically happening in the molecule.”
"Ultimately the question I want to pose to students is this,” said D’eon. "We have chemicals with varying global warming potential, with potential to produce varying levels of TFA. Which do you choose? It’s a discussion of balancing priorities and making difficult decisions.”